Carbon Compound Form 5
Chloramine removal by activated carbon is a much slower reaction. Carbon shows catenation due to its small size and Stronger carbon-carbon bond strength.
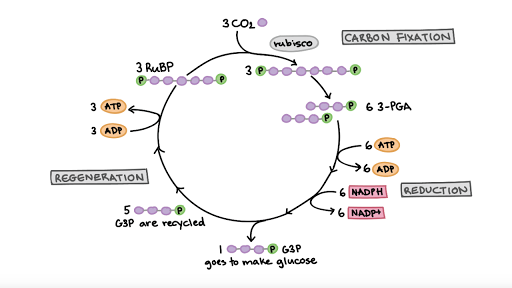
The Calvin Cycle Article Photosynthesis Khan Academy
The Ceramic Pourable Compound mixes and pours easily from the 5.
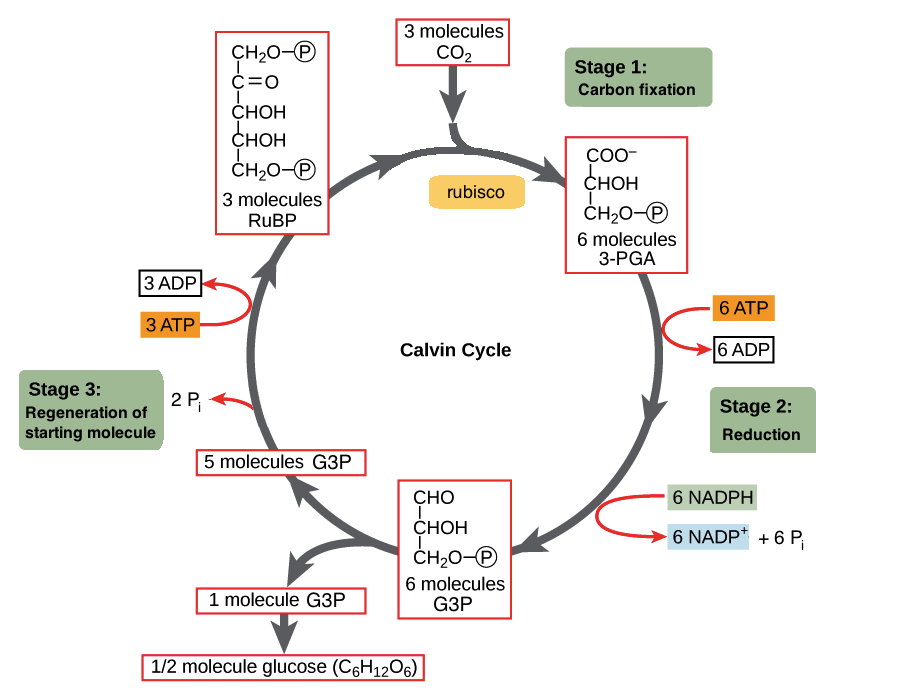
. Charcoal is an organic carbon compound. Write the name and molecular formula of the fourth member of alkane series. This property is called catenation.
Where removal of organics by activated carbon takes minutes removal of chlorine literally takes seconds. Product Dimensions 12 x 28 x 59 inches. Carbon has the unique ability to form bonds with other atoms of carbon giving rise to large molecules.
Compounds related to phosphine oxides include phosphine imides R 3 PNR and related chalcogenides. Other Technical Details. 30-inch Carbon Hunting Target Practice Arrows with Field Points High quality carbon target practice arrows for all bows.
Charcoal is widely used in outdoor cooking. Charcoal is generally obtained from the burning of plant parts like wood peat bones and cellulose. KESHES Archery Carbon Arrows for Compound Recurve Bows - 30 inch Youth Kids and Adult Target Practice Bow Arrow - Removable Nock Tips Points 12 Pack 46 out of 5 stars 1508.
The films are polycrystalline consisting of crystallites in the micron size range so lack the clarity and. Charcoal is produced by the incomplete combustion of plant and animal products. And a bed depth of 3 feet.
Item model number MX-4. It is a highly porous microcrystalline structure. Coordination compound any of a class of substances with chemical structures in which a central metal atom is surrounded by nonmetal atoms or groups of atoms called ligands joined to it by chemical bonds.
Item Weight 0141 ounces. Where B stands for the base. Most of the atom is empty space.
Coordination compounds include such substances as vitamin B12 hemoglobin and chlorophyll dyes and pigments and catalysts used in preparing organic. A carbanion is an anion in which carbon is trivalent forms three bonds and bears a formal negative charge in at least one significant resonance form. The rest consists of a positively charged nucleus of protons and neutrons surrounded.
Actually both graphite and diamond are initially formed but under these highly reactive conditions the graphitic deposits are etched off the surface leaving only the diamond. As such the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element.
Item Dimensions LxWxH 12 x 28 x 59 inches. Charcoal is mixed with clay to save energy in the brick formation. Atom smallest unit into which matter can be divided without the release of electrically charged particles.
Carbon-Core Corpformulates its Ceramic Pourable Compound with premium polyester resins and high strength ceramic spheres resulting in high tensile and flexural strength. Please enter a question. Phosphine oxides form hydrogen bonds and some are therefore soluble in water.
Formally a carbanion is the conjugate base of a carbon acid. R 3 CH B R 3 C. Please make sure that you are posting in the form of a question.
The chlorine capacity of new activated carbon is 1 pound of chlorine per pound of carbon at a flow rate of 3 to 5 gpmcuft. 1 offer from 3299. The carbon-based molecules then deposit on a surface to form a coating or thin film of diamond.
This lightweight compound is ideal for filling large volumes where strength and rigidity are major concerns. The PO bond is very polar with a dipole moment of 451 D for triphenylphosphine oxide. The carbanions formed from deprotonation of alkanes at an sp 3 carbon alkenes at an sp 2.
Phosphine oxides designation σ 4 λ 5 have the general structure R 3 PO with formal oxidation state V.

Lakhmir Singh Chemistry Class 10 Solutions Carbon And Its Compounds

Hydrocarbon Definition Types Facts Britannica
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

The Calvin Cycle Article Photosynthesis Khan Academy
0 Response to "Carbon Compound Form 5"
Post a Comment